 Last month, the DEA enthused the pharmaceutical industry but disappointed cannabis advocates by re-scheduling the drug Epidiolex—but not CBD, the cannabinoid that makes it work. Now word emerges of a letter to the DEA by the Food & Drug Administration essentially calling for the descheduling of CBD altogether.
Last month, the DEA enthused the pharmaceutical industry but disappointed cannabis advocates by re-scheduling the drug Epidiolex—but not CBD, the cannabinoid that makes it work. Now word emerges of a letter to the DEA by the Food & Drug Administration essentially calling for the descheduling of CBD altogether.

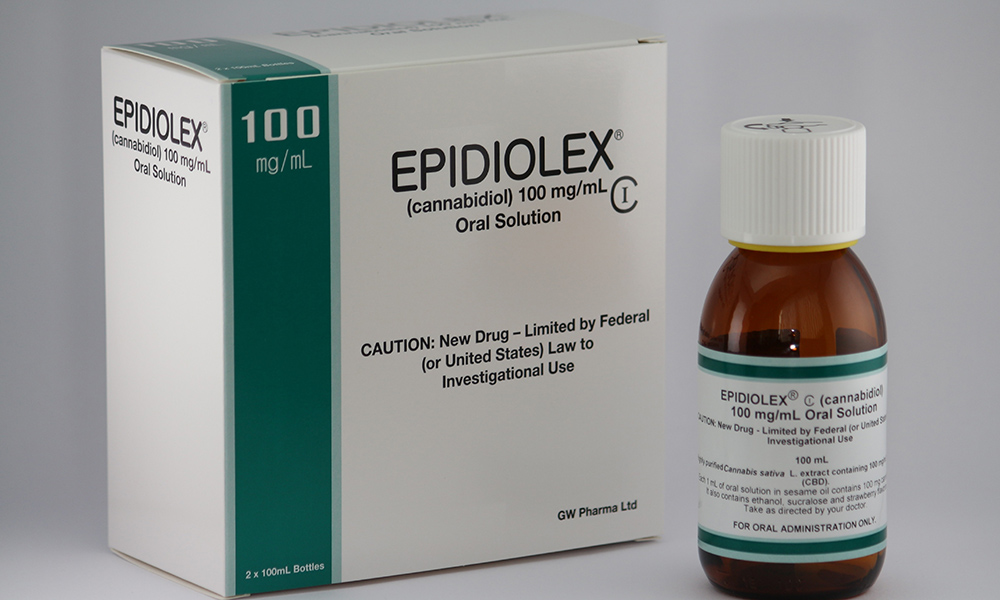 With the DEA's rescheduling of Epidiolex, shares in the British manufacturer of the drug are soaring. But CBD—the actual cannabinoid that the product is based on—is to remain in the restrictive Scheudle 1.
With the DEA's rescheduling of Epidiolex, shares in the British manufacturer of the drug are soaring. But CBD—the actual cannabinoid that the product is based on—is to remain in the restrictive Scheudle 1.
 Among several cannabis-related bills that cleared California's state house before the last legislative session came to close is one that would lift the tax burden on medical marijuana providers. The bill is intended to again open space for "compassionate care," which was ironically squeezed out under California's adult-use regulation regime.
Among several cannabis-related bills that cleared California's state house before the last legislative session came to close is one that would lift the tax burden on medical marijuana providers. The bill is intended to again open space for "compassionate care," which was ironically squeezed out under California's adult-use regulation regime.  Having just undertaken a "critical study" of CBD, the non-psychoactive cannabinoid held to have multifarious medicinal applications, the World Health Organization is now opening such a study on THC. Stigmatized due to its psychoactive properties and currently in the shadow of the suddenly sexy CBD, tetrahydrocannabinol shows its own potential for application by the medical industry.
Having just undertaken a "critical study" of CBD, the non-psychoactive cannabinoid held to have multifarious medicinal applications, the World Health Organization is now opening such a study on THC. Stigmatized due to its psychoactive properties and currently in the shadow of the suddenly sexy CBD, tetrahydrocannabinol shows its own potential for application by the medical industry. The British government has finally capitulated to pressure from patients, activists and even regional authorities, and officially pledged to make medical cannabis products available on prescription this year. But it remains to be seen which products will win approval—and actual herbaceous cannabis is unlikely to make the cut.
The British government has finally capitulated to pressure from patients, activists and even regional authorities, and officially pledged to make medical cannabis products available on prescription this year. But it remains to be seen which products will win approval—and actual herbaceous cannabis is unlikely to make the cut. A federal appeals court has turned down a challenge by the cannabis industry to the DEA's official finding that CBD is a controlled substance. But questions about whether CBD should be treated as a controlled substance remain pending at the state level.
A federal appeals court has turned down a challenge by the cannabis industry to the DEA's official finding that CBD is a controlled substance. But questions about whether CBD should be treated as a controlled substance remain pending at the state level.





Recent comments
2 weeks 6 days ago
2 weeks 6 days ago
6 weeks 11 hours ago
6 weeks 6 days ago
11 weeks 31 min ago
14 weeks 5 days ago
18 weeks 5 days ago
19 weeks 3 days ago
29 weeks 3 days ago
33 weeks 4 days ago